Owing to hydrogen’s high energy density, solar water splitting for green hydrogen production has emerged as a focal point of research interest. In this context, a collaborative team from Macau University of Science and Technology (M.U.S.T.), Hong Kong, and Saudi Arabia reported the discovery of quantum confinement effects in a three-dimensionally ordered macroporous photocatalyst. The observed quantum confinement and cocatalyst decoration facilitate hydrogen and oxygen generation under visible light irradiation, offering new insights into photocatalyst design for sustainable energy applications.
The study was co-led by Dr Hao Wu, Assistant Professor at the Department of Materials Science and Engineering (DMSE), Faculty of Innovation Engineering (FIE), M.U.S.T., in collaboration with researchers from Hong Kong and Saudi Arabia. The findings were published in the Journal of American Chemical Society titled “Bismuth Vanadate Capable of Driving One-Step-Excitation Photocatalytic Overall Water Splitting”.
Dr Wu, an expert in photocatalysis research, pointed out that using sunlight to directly split water in a single step is one of the simplest and most promising methods for large-scale hydrogen production. Metal sulfide photocatalysts can split water using visible light and show great potential for hydrogen production. However, they often break down because the sulfur inside them reacts with light and oxygen, causing damage—a problem known as photocorrosion.
Although semiconducting oxides such as bismuth vanadate (BiVO4) are good at absorbing visible light and are also stable, they cannot split water on their own. This is because their energy level is too low to produce hydrogen, and they also suffer from serious energy loss due to electrons and holes quickly recombining. In these regards, Dr Wu and his team took on this challenge and investigated the marriage of quantum confinement effects and cocatalyst decoration in achieving one-step-excitation photocatalytic overall water splitting to produce hydrogen and oxygen.
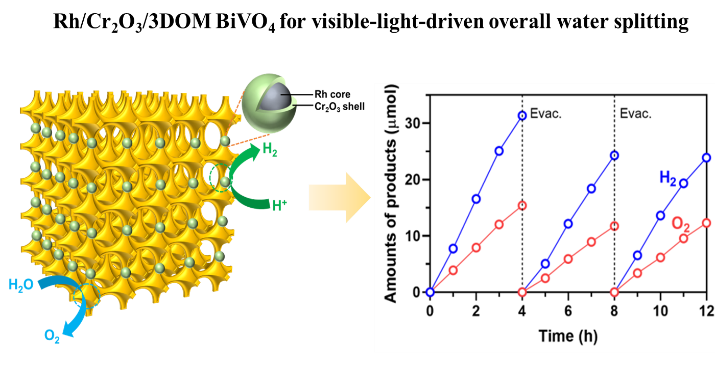 A highly ordered three-dimensional macroporous (3DOM) BiVO₄ photocatalyst with very thin walls and good crystal quality was synthesized from Dr Wu’s laboratory and this special structure showed a quantum confinement effect, which raised the energy level of its conduction band. As a result, it was able to produce hydrogen under visible light when sacrificial agents were added to help the reaction. However, even though the uplifted energy level was enough to generate hydrogen, the 3DOM BiVO4 still couldn’t split pure water into hydrogen and oxygen in the right ratio. This is because the reaction faces higher kinetic barriers and the charges inside the photocatalyst tend to recombine before they can finish the water-splitting process. To solve this problem, Dr Wu and his team further loaded hydrogen evolution cocatalyst of Rh/Cr2O3 on the 3DOM BiVO4 surface and finely tuned the reaction conditions, successfully demonstrating a one-step-excitation photocatalytic overall water splitting, which evolves hydrogen and oxygen in a stoichiometric ratio under visible light irradiation with an apparent quantum yield (AQY) of 0.47% at 400 nm.
A highly ordered three-dimensional macroporous (3DOM) BiVO₄ photocatalyst with very thin walls and good crystal quality was synthesized from Dr Wu’s laboratory and this special structure showed a quantum confinement effect, which raised the energy level of its conduction band. As a result, it was able to produce hydrogen under visible light when sacrificial agents were added to help the reaction. However, even though the uplifted energy level was enough to generate hydrogen, the 3DOM BiVO4 still couldn’t split pure water into hydrogen and oxygen in the right ratio. This is because the reaction faces higher kinetic barriers and the charges inside the photocatalyst tend to recombine before they can finish the water-splitting process. To solve this problem, Dr Wu and his team further loaded hydrogen evolution cocatalyst of Rh/Cr2O3 on the 3DOM BiVO4 surface and finely tuned the reaction conditions, successfully demonstrating a one-step-excitation photocatalytic overall water splitting, which evolves hydrogen and oxygen in a stoichiometric ratio under visible light irradiation with an apparent quantum yield (AQY) of 0.47% at 400 nm.
The Macau University of Science and Technology team investigated the quatum confinement effect of 3DOM BiVO4 and decorated it with cocatalyst to produce hydrogen and oxygen under visible light excitation.
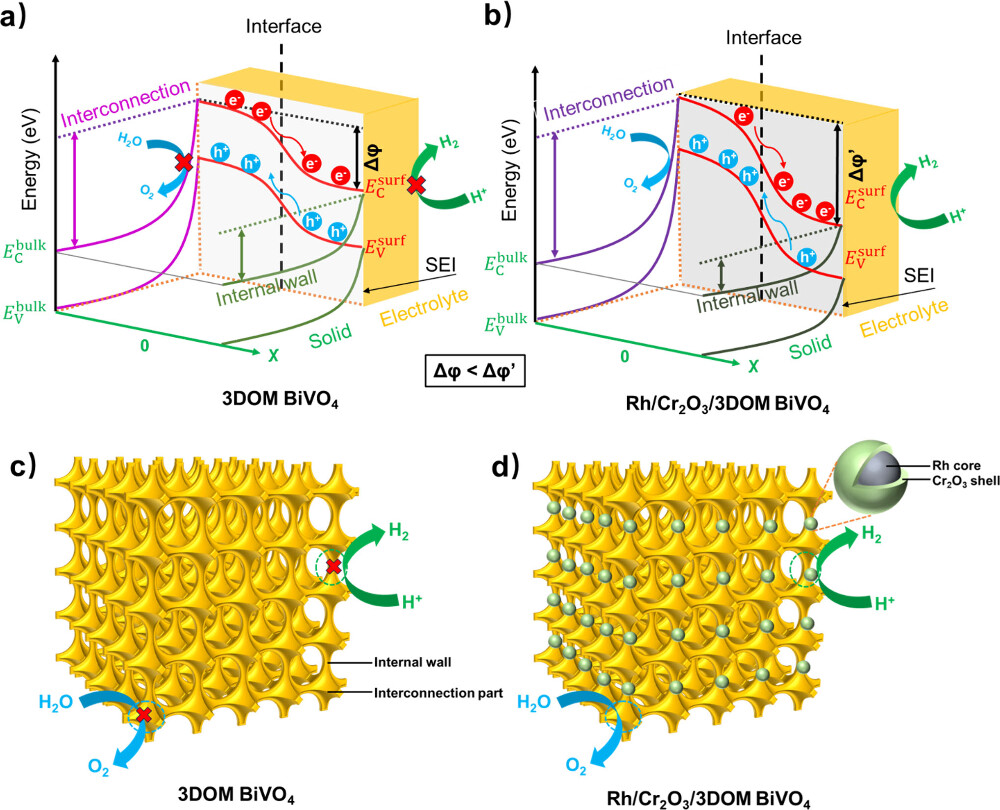 The team also applied advanced techniques, including Kelvin probe force microscopy, to investigate the electrical potential distribution in the Rh/Cr2O3-decorated 3DOM BiVO4 photocatalyst by spatially resolved surface photovoltage. The results suggest that the surface potential difference between the interconnection and the internal wall of the 3DOM BiVO4 has increased from ~25 mV to ~50 mV upon the deposition of Rh/Cr2O3 cocatalyst. It indicates that the band bending within the space charge region near the interconnection surface of 3DOM BiVO4 steepened, while that near the internal wall surface flattened, thus intensifying the internal built-in electric field between the interconnection and the internal wall.
The team also applied advanced techniques, including Kelvin probe force microscopy, to investigate the electrical potential distribution in the Rh/Cr2O3-decorated 3DOM BiVO4 photocatalyst by spatially resolved surface photovoltage. The results suggest that the surface potential difference between the interconnection and the internal wall of the 3DOM BiVO4 has increased from ~25 mV to ~50 mV upon the deposition of Rh/Cr2O3 cocatalyst. It indicates that the band bending within the space charge region near the interconnection surface of 3DOM BiVO4 steepened, while that near the internal wall surface flattened, thus intensifying the internal built-in electric field between the interconnection and the internal wall.
Cocatalyst loading tuned the surface band bending of 3DOM BiVO4 photocatalyst and promoted spatial charge separation.
The next goal of Dr Wu and his team is to apply diverse strategies such as doping, cocatalyst modification, and dimension control to further improve the performance and explore methods to scale up solar water splitting systems. “Green hydrogen produced from solar water splitting is a sustainable process with negligible carbon emissions,” said Dr Wu. “Hydrogen serves as a clean energy carrier for industrial applications and fuel cells for electricity generation. Given the growing demand for sustainable hydrogen production, this technology is expected to see broader adoption in the future.”
Dr Wu and Prof Yun Hau Ng, from the King Abdullah University of Science and Technology (KAUST), are the corresponding authors of the paper. The first author is Dr Wu. Dr Songying Qu, a Postdoc in the FIE of M.U.S.T. also participated in the research. This research was supported by the Science and Technology Development Fund, Macao SAR, and the National Natural Science Foundation of China.
DOI number: doi.org/10.1021/jacs.4c18733





